SECOND LAW OF THERMODYNAMICS
- KELVIN PLANK STATEMENT: - It is impossible for a heat engine working on cyclic process which can convert all the heat supplied to it into equivalent amount of mechanical work.
- CLAUSIUS STATEMENT: - It is impossible for a self acting machine, working in a cyclic process, to transfer heat from a body at lower temperature to a body at higher temperature without the external aid.
Work is a high grade of energy and Heat is a low grade of energy.
- EFFICIENCY OF HEAT ENGINE: -
A heat engine works on a thermodynamic cycle.
The efficiency is the ratio of net work transfer to the net heat transfer to the system.
ηth = Wnet/ Q1 = (Q1 - Q2) / Q1
Where Q1 = Heat flow from source to the heat engine.
and Q2 = Heat rejected from heat engine to the sink.
ηth = (TH– TL) / TH
=(1- TL/ TH)
Where TH = Higher Temperature
and TL = Lower Temperature
- COEFFICIENT OF PERFORMANCE OF REFRIGERATOR: -
It is defined as the ratio of Refrigerating Effect to the work input.
 |
| Fig.2 | COP of Refrigerator |
COPR = Q2/ (Q1 – Q2) [FOR BOTH REVERSIBLE AND IRREVERSIBLE]
COPR (REV. CYCLE)= TL / (TH – TL) = (1 – ηth) / ηth
- COEFFICIENT OF PERFORMANCE OF HEAT PUMP: -
It is defined as the ratio of Heating Effect to the work input.
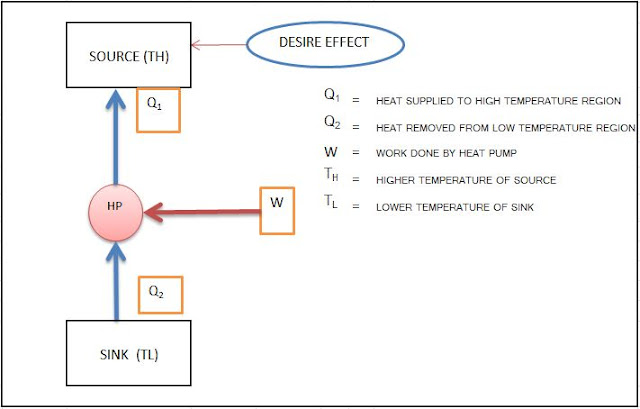 |
| Fig.3 | COP of Heat Pump |
COPHP = Q1/ (Q1 – Q2) [FOR BOTH REVERSIBLE AND IRREVERSIBLE]
COPHP (REV. CYCLE)= TH / (TH – TL) =1 / ηth
- COPHP = 1+ COPR
- PERPETUAL MOTION MACHINE OF SECOND KIND (PMM II): -
The machine which can continuously absorb heat from a single thermal reservoir and convert this completely into work.
The thermal efficiency of search machine is 100%
This is impossible.



2 Comments
This comment has been removed by a blog administrator.
ReplyDeleteThanks For sharing this Superb article.I use this Article to show my assignment in college.it is useful For me Great Work. broward criminal lawyers
ReplyDeletePlease do not enter any spam link in the comment box